What are biogeochemical cycles?
Nearly 30 to 40 elements are required for the proper growth and development of living organisms. Most important of these are C, H, O, P, K, N, S, Ca, Fe, Mg, B, Zn, Cl, Mo, I, and F. these materials flow from abiotic to biotic components and back to the non-living component again in a more or less cyclic manner. This is known as the biogeochemical cycle or inorganic-organic cycle. The flow of these elements through the ecosystem must be cyclic, with the matter being constantly reused. Because the flow involves living organisms and a series of chemical reactions in the abiotic environments, these cycles are called biogeochemical cycles.
Types of biogeochemical cycles
There are three types of biogeochemical cycles:
- Hydrologic cycle or water cycle
- Gaseous cycle:
- Oxygen cycle
- Carbon cycle
- Nitrogen cycle
- Sedimentary cycle:
- Sulfur cycle
- Phosphorous cycle
Hydrologic or water cycle:
Interchange of water between atmosphere, land, and sea and between living organisms and their environment is accomplished through the water cycle. The water cycle or hydrologic cycle involves evapotranspiration, condensation, precipitation, infiltration, throughflow, runoff, groundwater flow, and evaporation.
The key process is evaporation since it maintains the humidity of the atmosphere and feeds back the moisture necessary for cloud formation and precipitation. The atmosphere’s water reaches the earth’s surface through precipitation, and from the earth’s surface, it reaches the atmosphere through evaporation and transpiration. The amount of water available for evaporation is determined by the amount supplied by precipitation and condensation. Between rainfall input and evaporation output, there lies a precarious water balance.
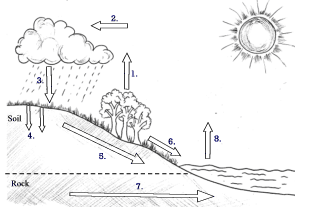
Fig: Hydrological cycle in nature
Oxygen cycle:
Oxygen is found in the free state of the atmosphere and dissolved in water. It is liberated as a byproduct of photosynthesis and is utilized in respiration by all plants and animals. How plants release oxygen in the atmosphere is given below:
When the living organisms respire, it is liberated, which is utilized by green plants as an essential raw material for carbohydrates synthesis. In this way, a simple yet vital O2 cycle is maintained in the ecosystem.
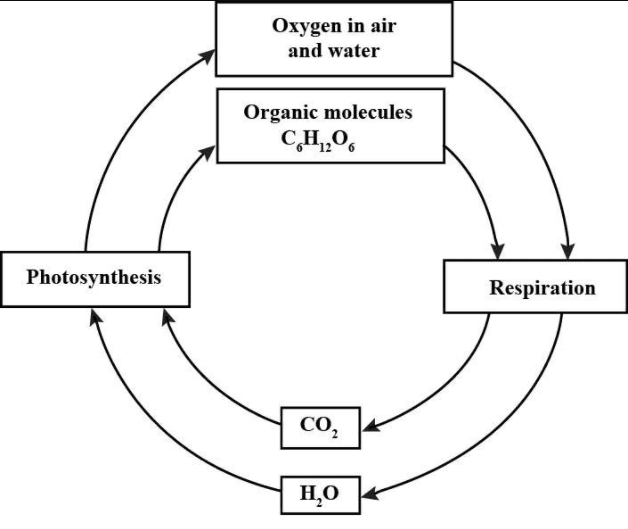
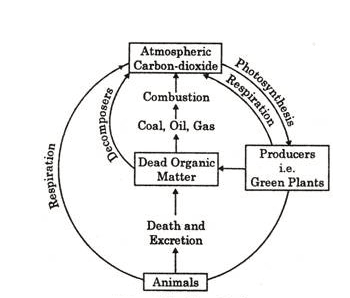
Carbon cycle:
Carbon is the basic constituent of all organic compounds, e.g., carbohydrates, proteins, fats, etc. Since energy transfer occurs in the consumption and storage of carbohydrates and fats, carbon moves to the ecosystem with energy flow. The source of nearly all carbon found in living organisms is CO2 found in the free state in the atmosphere and dissolved in the water on the earth.
The amount of CO2 present in the air is 0.03%, but this amount is 50 times higher than that of the air in the sea. This CO2 of the sea controls the amount of CO2 in the air. Carbon constitutes 40-50% of the dry weight of plants. Green plants (producers) use CO2 through photosynthesis in the presence of sunlight and carbohydrate is formed. Later on, complex fats and polysaccharides are formed in plants which are utilized by animals. Carnivores feed on herbivores, and the carbon compounds are again digested and converted into other forms.
Carbon is released to the atmosphere directly as CO2 in the respiration of both plants and animals. Bacteria and fungi attack the dead remains of plants and animals. They degrade the complex organic compounds into simple substances, which are then available for other cycles. Part of the organic carbon becomes incorporated into the earth’s crust as coal, gas, petroleum, limestone, and coral reef. Carbon from such deposits may be liberated after a long period.
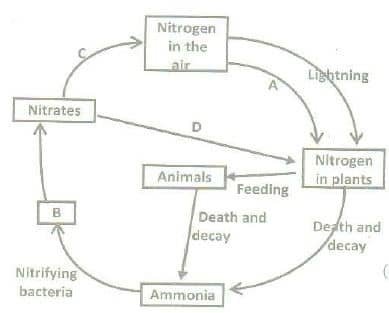
Nitrogen cycle:
All the elements that plants absorb from the soil are most important for plant growth. This is required in the greatest quantity. Green plants obtain nitrogen from the soil solution in ammonium or nitrate or nitrite ions. The most important source of nitrogen for green plants is nitrogen that is fixed by nitrogen-fixing bacteria. Some of the nitrogen-fixing bacteria inhabit the root nodules of leguminous and some other plants, and some others are free in the soil.
Nitrogen is directly taken from the air by nitrogen-fixing nodule bacteria (rhizobia) or by free-living aerobic bacteria (Azotobacter), or anaerobic soil bacteria (Clostridium). Those bacteria make nitrogen available to the plants and add to their growth. Some blue-green algae, as for example, species of Nostoc and Anabaena, also perform nitrogen fixation. When nitrogen is absorbed as nitrate, it must be reduced to ammonia before being used in amino acid and protein synthesis.
The breakdown of dead tissues by decay bacteria releases ammonia from protein and other nitrogenous compounds. Nitrosomonas bacteria oxidize ammonia into nitrites, and still other bacteria (Nitrobacter) oxidize nitrite to nitrate (Nitrification). Ammonia is also directly converted into free nitrogen by denitrifying bacteria (Denitrification). In this way, the nitrogen cycle is repeated in the ecosystem.
A: Nitrogen fixation, B: Nitrites, C: Denitrification, D: Plant assimilation
Sedimentary cycle:
Mineral elements required by living organisms are obtained initially from inorganic sources. Available forms occur as salts dissolved in soil water. The mineral cycle essentially consists of two processes: 1. The salt solution phase, and 2. Rock solution phase.
Mineral salts come directly from the earth’s crust by weathering. Soluble salts then enter the water cycle. By water movement, minerals move from the soil to streams, lakes, and ultimately to the sea, where they remain permanently. Other slats return to the earth’s crust through sedimentation. They become incorporated into sediments or beds, and after weathering of rocks, they again enter the cycle
Plants and some animals take minerals in the form of mineral solutions from their habitats. After the death of living organisms, the minerals return to the soil and water through the action of decomposers (bacteria and fungi) and transformers. Green plants at one end and decomposers at another play a significant role in the circulation of nutrients. Two major types of the sedimentary cycle are found in nature:
Phosphorus cycle:
Phosphorus (mainly phosphate ion) is an essential nutrient for plants and animals. It is an essential constituent of nucleic acid, phospholipid, ATP, cell membrane, teeth, and bone.
The phosphorus cycle is not related to the atmospheric phase. The source of this cycle is rocks (sediments) and other matters where phosphorus had been accumulated a long time ago. Phosphorus-containing rocks supply phosphorus slowly by decaying due to many natural processes taking place. A portion of phosphorus gets deposited in riverbeds, and the bottom of oceans being washed away with water. Some are deposited in the deep bottom of the oceans, and some get deposited in the shallow bottom of oceans.
Plants obtain phosphorus from the environment, and animals obtain phosphorus by taking plants as food. When plants and animals die, the decomposers (phosphate solubilizing bacteria and others) attack them and liberate phosphorus from the environment. Phosphorus comes to soil and water directly or indirectly from excretory materials and debris of teeth and bones of animals. Guano, the excretory product of fish-eating birds (Pelican, Gannet, etc.), contains phosphorus, and phosphorus is transferred to the soil. This process proceeds cyclically.
Phosphorus, calcium, and many other elements also reach the oceans in a dissolved state, and there they undergo sedimentation (accumulation). In the sedimentary deposits, the nutrients are locked up for an indefinite period, and in this way, they are separated from the cyclic pathway. When sediments weather, the nutrients become free for another cycle.

Sulfur cycle:
Both the atmospheric phase and soil phase are related to the Sulfur cycle. Sulfur is an essential element for living bodies. It is an essential constituent of some particular amino acids, e.g., cystine, cysteine, and methionine, vitamins like biotin and thiamine. It is not as much required as nitrogen and phosphorus are required to grow and reproduce plants.
Sulfur is found in many forms in nature. Like Sulphur (S), sulfide, Sulfur mono-oxide, and sulfate (SO4). Sulfur is heavily used to produce many chemical compounds, gun powder, sulfuric acid (H2SO4), and fertilizers. When living organisms die, Sulfur is freed into the soil in different forms by decomposers like bacteria. In the absence of oxygen, hydrogen sulfide (H2S) is formed. But in oxygen, it is converted to sulfate (SO43-), which is taken by plants. Anaerobic bacteria use H2S for their respiration. These bacteria live in the depth of oceans or anaerobic regions of eutrophic lakes and form sulfate compounds from H2S.
Sulfur dioxide (SO2) is freed in the air by burning coal and petroleum. From mill and factories, 1/3 of Sulfur is freed in the air as Sulfur dioxide (SO2). SO2 reacts with oxygen (O2) to form Sulfur trioxide (SO3), which comes to the soil in the form of sulfuric acid (H2SO4) as rain (reacting with rainwater), and this is called acid rain.







No comments:
Post a Comment